There are four fundamental forces;
- gravity
- electromagnetic force
- strong nuclear force
- weak nuclear force
The protons in a nucleus are all positively charged and so they repel each other (this is the electromagnetic force in action). This should push the protons apart but it doesn’t so there must be another force which keeps the nucleus together. This force is called the STRONG NUCLEAR FORCE.
The strong nuclear force
The strong nuclear force holds neutrons and protons (nucleons) together in the nucleus.
Hadrons (mesons & baryons) experience the strong nuclear force but leptons do not.
The strong nuclear force acts over a very short range. It can be both attractive & repulsive.
- It gives rise to short range attraction between adjacent nucleons, up to a distance of about 3 x 10-15m. (or 3 femtometers).
- It also gives rise to very short range repulsion below 0.5 x 10-15m(or 0.5 femtometers).
Stable & Unstable Nuclei
Bismuth with a proton number (z) of 83 is the stable nuclei with the highest number of protons. All nuclei with proton numbers above 83 are unstable, they are RADIOACTIVE.
The radioactive elements emit;
- alpha particles (a) – 2 protons and 2 neutrons (helium nucleus)
- beta particles (b) – a high speed electron
- gamma rays (g) – a photon
Alpha decay (a)
The radioactive parent nuclide decays into a new lighter daughter nuclide by emitting and alpha particle.
Example; the parent nuclide thorium-232 decays into the daughter nuclide radium-228.
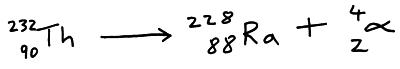
The proton and nucleon numbers on each side of the decay equation must balance. So if X decays into Y. The nucleon number of Y must be 4 less than X, and the proton number of Y must be 2 less than X.
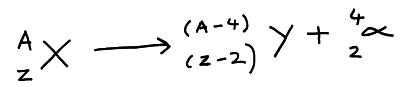
Beta decay (b–)
A beta–particle is produced when a neutron in the parent nuclide decays into a proton by emitting a beta– particle and an antineutrino.
Example; carbon-14 decaying into nitrogen-14
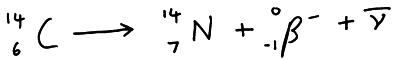
When the parent decays into the nucleus the nucleon number stays the same BUT the proton number is one less in Y then it was in Z.
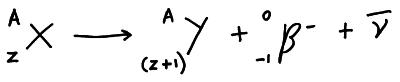
Neutrino
An antineutrino is emitted in the above decay, this is the antiparticle of the neutrino. These particles have no charge and nearly zero mass.
Gamma Radiation (g)
When nuclide emits a photon of electromagnetic radiation it is called a gamma (g) ray.
Links to other pages in this topic;



