Radioactive decay occurs because a nuclei is unstable. It emits alpha particles, beta particles or gamma rays to become more stable.

Z is the proton number (sometimes called atomic number) it is the number or protons in the nucleus.
A is the nucleon number (some times called mass number) it is the total number of protons AND neutrons in the nucleus.
N is the number of neutrons = A – Z
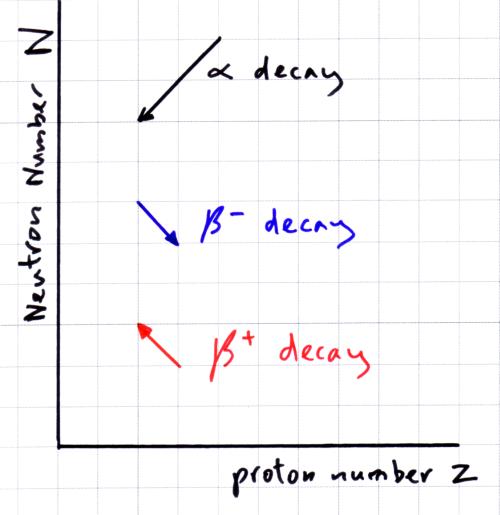
Graph of N against Z.

- for light isotopes (up to Z=20) the stable nuclei have equal numbers of protons and neutrons
- for heavier nuclie to be stable they need to have more neutrons than protons (see the line for stable isotopes on the graph above)
- nuclei which are unstable lie either side of the stability line
- nuclei with too many protons are to the right of the stability line and become more stable by emitting a beta+ (b+) particle when a proton converts into a neutron
- nuclei with too few protons are to the left of the stability line and become more stable by emitting a beta- (b–)particle when a neutron converts into a proton
How nuclei change when they decay
Alpha decay
In alpha decay the unstable parent nuclei emits an alpha particle (2 protons and 2 neutrons) so the daughter nuclei has 2 less protons and 2 less neutrons.
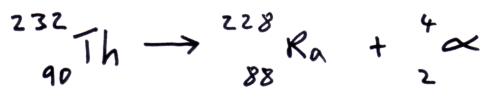
Beta- (b–) decay
In the parent nuclei a neutron changes into a proton and emits a beta- b– particle (electron). So the daughter nuclei has one less neutron and one more proton.
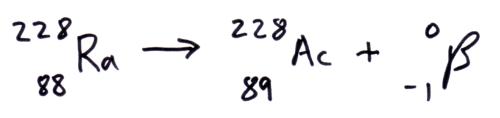
Beta+ (b+) decay
In the parent nuclei a proton changes into a neutron and emits a beta+ b+particle (positron). So the daughter nuclei has one less proton and one more neutron.
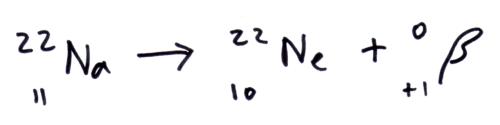
Electron capture
In electron capture a proton captures and orbiting electron and the combines to form a neutron. So the daughter nuclei has one less proton and one more neutron.
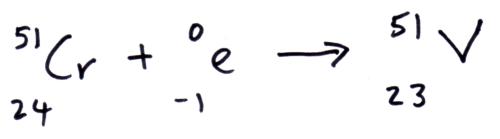
Gamma ray emission
Gamma ray emission may happen at the same time as alpha or beta decay or it may occur a short while after. The nuclei becomes more stable in gamma ray emission by emitting a photon. There is no change in the number of protons or neutrons when a gamma ray is emitted.

Technetium-99m (the ‘m’ refers to the nucleus being in a metastable state) is used in medical diagnosis as a gamma ray sources because it has a half life of 6 hours which is a gives enough time for the measurements to be taken in hospital before the activity of the technetium decays away.



