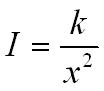Alpha (a) decay
An alpha particle is 2 protons and 2 neutrons (i.e. a helium nucleus). When an atom emits an alpha particle its proton number reduces by 2 so it becomes a different element. Alpha particles are positively charged and so can be deflected by electric and magnetic fields.
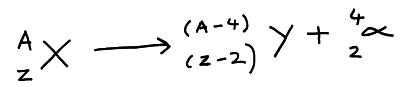
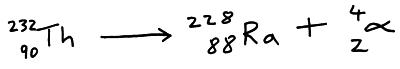
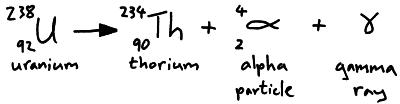
Beta (b–) decay
A b– particle is an electron so it is negatively charged so it can be deflected by electric and magnetic fields. During b– decay a neutron is converted into a proton and an electron and an antineutrino. This increases the proton number by one making the atom a different element.
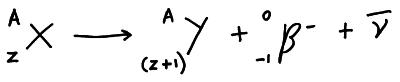
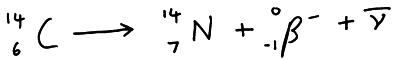
Gamma (g) radiation
Alpha and beta decay can produce a daughter nucleus in an excited state. The daughter nucleus can decay by emitting a gamma ray (i.e. a photon of electromagnetic radiation).
Gamma rays have no charge and so are unaffected by electric and magnetic fields.
Identification using simple absorption experiments.
Alpha, beta and gamma radiation can be identified using the variation in their penetration;
- alpha particles are stopped by a few cm of air or a thin sheet of paper
- beta particles are stopped by a few mm of aluminium
- gamma ray intensity is halved by 10cm of lead
Identification using simple electric or magnetic fields.
- alpha particles are deflected in the same direction as other positively charged particles
- beta particles are deflected in the opposite direction to alpha particles
- gamma rays are not deflected at all
Safe handling of radioactive sources
- always handle with long (30cm) tweezers
- always return to the container when finished with to limit exposure time
- point sources away from the body and handle at arms length
Relative hazards of exposure to humans
Alpha, beta and gamma radiation can ionise atoms in the body. Alpha particles are the most ionising and gamma rays are the least ionising. Alpha particles are the least penetrating and gamma rays are the most penetrating.
- alpha particles outside the body can be stopped by cloths / skin and are relatively harmless, alpha particles inside the body are very harmful as they are the most ionising
- outside the body beta particles and gamma rays are more harmful than alpha particles as the can penetrate into the body.
Background radiation
Radiation detectors detect radiation even when no radioactive sources are present. This is because of background radiation which is always present. Background radiation comes from;
- cosmic rays
- natural radioactive material in rocks / soil
- radon gas
- a small amount comes from medical sources, nuclear power stations and nuclear test
Before an experiment the background radiation should be measure so that it can be deducted from the measurements made in the experiment to correct for background radiation.
Inverse square law for g radiation
If you double the distance from a gamma ray source then the intensity of the radiation will be one quarter what it was before (this is an inverse square law).