Gas laws
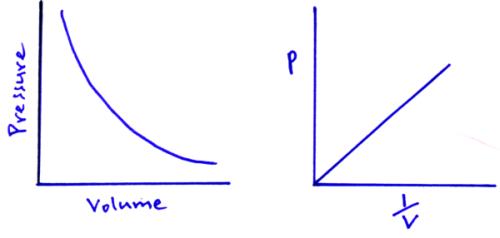
Boyle’s law
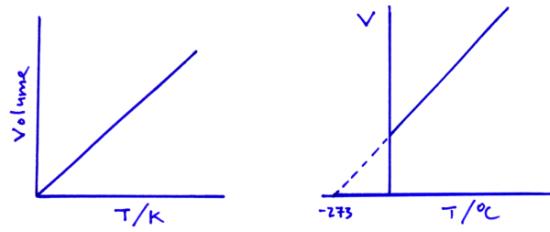
Charles’ law
Pressure law
Ideal gas equation
P = pressure of the gas (Pa)
V = volume of the gas (m3)
n = number of moles
R = molar gas constant 8.31 J kg-1mol-1
T = temperature of the gas (K)
The equation above can be rewritten in terms of the number of molecules (N) in the gas rather than the number of moles (n), see below.
P = pressure of the gas (Pa)
V = volume of the gas (m3)
N = number of molecules in the gas
K = Boltzmann constant 1.38 x 10-23 J K-1
T = temperature of the gas (K)
Other terms mention in the specification.
number of moles (n)
One mole is the amount of of a substance which contains the same number of molecules as there are in 12 g of Carbon-12. The number of molecules is called the Avogadro constant NA = 6.02 x 1023 mol-1
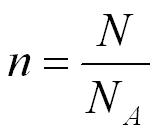
N = number of molecules in the gas
NA = Avogadro constant 6.02 x 1023 mol-1
molar mass (Mm)
The molar mass of a substance is the mass of one mole of the substance.
molecular mass (m)
The molecular mass of a substance is the mass of one molecule of that substance.