In this work temperature is measured in kelvin (K) to convert a temperature in degrees Celsius (oC) just add on 273.
For example a temperature of 35 oC is 35 + 273 = 308 K
Specific heat capacity (c)
The specific heat capacity of a material is the amount of energy (Q) needed to raise 1 kg of a material through a temperature change of 1 kelvin.
Q = energy in / or out of the material in joules (J)
m = mass of the material in kilograms (kg)
c = the specific heat capacity of the material in J kg-1 K-1
DT = change in temperature in kelvin (K)
Latent heat (L)
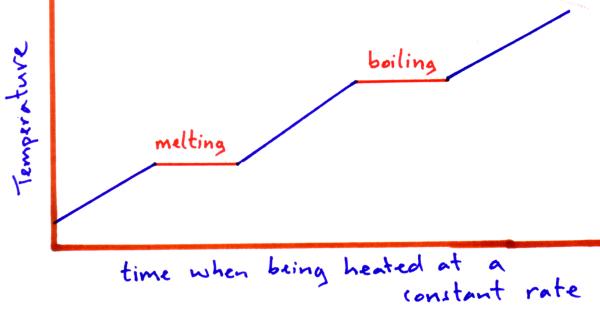
Latent heat is taken in / or given out when a material changes state e.g. solid to liquid or liquid to gas.
![]()
Q = energy in / or out of the material in joules (J)
m = mass of the material which has changed state in kilograms (kg)
L = the latent heat in J kg-1
When a material goes from solid to liquid or liquid to solid it is called the latent heat of fusion.
When a material goes from a liquid to gas or gas to a liquid it is called the latent heat of vaporisation.