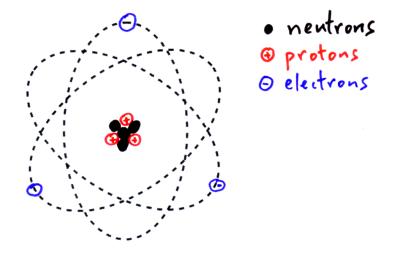
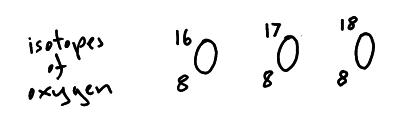Atoms
Atoms have a central nucleus which contains protons and neutrons and is surrounded by electrons.
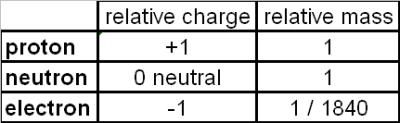
Mass number and atomic number
The total number or protons + neutrons is called the mass number (sometimes called the nucleon number in Physics).
The number of protons in an atom is called the atomic number (sometimes called the proton number in Physics).
Below is Lithium (Li), it has 3 protons in the nucleus and 4 neutrons.
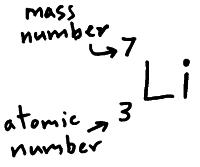
Ions
Most atoms have no overall electric charge, they are neutral because they have equal numbers of positive (+) protons and negative (-) electrons.
Atoms can gain electrons to become negative ions or lose electrons to become positive ions.
Charge attraction & repulsion
Charged particles which have the same charges ( + + ) or ( – – ) repel each other.
Charged particles which have different charges ( + – ) attract each other.
Isotopes
Atoms of the same element always have the same number of protons ( is it had a different number of protons it would be a different element).
However atoms of the same element can have different numbers of neutrons (this gives them a different mass number). Different versions of the same element are called isotopes. Below are isotopes of oxygen, they all have the same number of protons but different numbers of neutrons.