Some materials are radioactive, they give out radiation from their nucleus. When the radioactive atoms give out the radiation is random and its rate cannot be affected by heat and pressure etc.
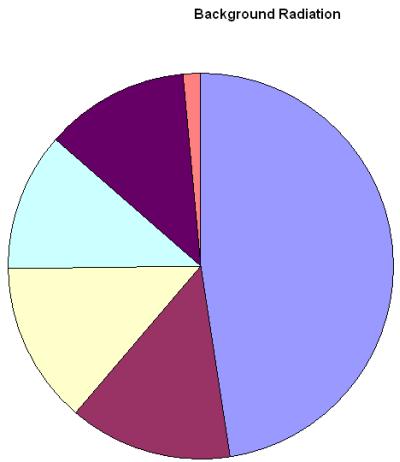
Background Radiation
We are surrounded by radiation all the time some of it is naturally occurring in radioactive rocks and cosmic rays and some of it is man made and was generated in nuclear tests and accidents. The radiation which occurs in the environment and we cannot avoid is called background radiation.

There are three types of radiation which can be emitted from the nucleus of an atom. Alpha particles, beta particles and gamma rays.
Alpha particles
Alpha particles are two protons and two neutrons (a helium nucleus) they are emitted from the nucleus of an atom. We can represent alpha particle emission in a nuclear equation.
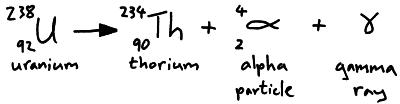
Beta particles
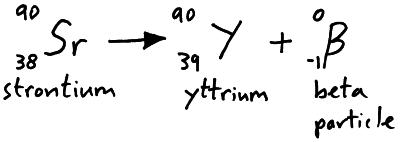
Beta particles are high speed electrons emitted from the nucleus of an atom when a neutron decays into a proton. We can represent beta particle emission in a nuclear equation.
Gamma rays
Gamma rays are an electromagnetic wave emitted from the nucleus of an atom.
Half-life
The half life is the time taken for half the radioactive isotope nuclei to decay.
OR
The half-life is the time for the count rate to fall to half its initial level.
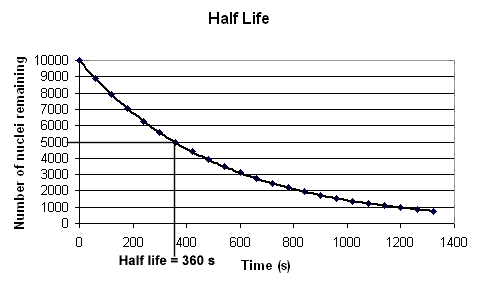
In the above example we can see from the graph it takes 360 seconds for the number of nuclei remaining un-decayed to fall from 10000 to 5000. So the half life for this radioactive isotope is 360 s.
The half-life for different radioactive isotopes can vary from fractions of a second to hundreds of thousands of years.



