Energy can be transferred by conduction, convection, evaporation and condensation. All these processes involve particles.
Conduction
Is the movement of thermal energy through a material without the particles in the material moving.
Glass, wood and plastic are all poor conductors of heat.
Heat is the kinetic energy of particles as they vibrate. If we heat the particles at one end of a material the particles at that end vibrate more (have more kinetic energy) and bump into the neighbouring particles which causes them to vibrate more. They collide with their neighbours and so the energy is passes from one particle to another through the material.
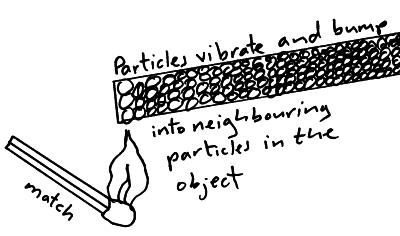
Metals are very good conductors of heat energy because there is a second process at work. Metals have free electrons, the free electrons gain extra kinetic energy at the warm end of the material. They diffuse to cooler areas where they collide with the particles in the material transferring their kinetic energy.
Convection
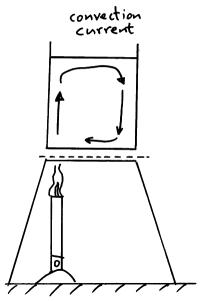
Convection happens in fluids (liquids & gases). The heat energy moves through the material as the particles in the material move through the material.
Hot liquids and gases rise because when they are heated they expand and become less dense. The less dense warm liquid or gas then floats up through the more dense cold liquids and gases.
Cold liquids and gases sink because when cooled they contract and become more dense. The more dense cold liquids and gases sink down through the less dense warm liquids and gases.
These changes in density cause the convection currents in the liquid or gas.
Click link to thermal radiation.
Evaporation
In a liquid the particles have a range of energies. At the surface of the liquid some particles will have enough energy to escape from the liquid and overcome the attraction of the other liquid particles. This leaves the less energetic particles still in the liquid and so the liquid is cooler.
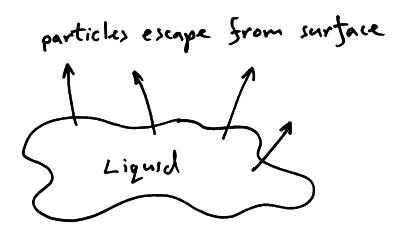
To make a liquid evaporate more quickly you could;
- increase the temperature of the liquid, so more of them have enough energy to escape the liquid
- increase the surface area of the liquid
- pass air across the surface of the liquid
Condensation
Condensation is the change of state from a gas to a liquid, it is caused by cooling. For example water vapour in warm air condenses into a liquid when cooled.



