Radioactive decay is a random process. You cannot predict when an individual nucleus will decay but with large numbers of nuclei you can use a statistical approach.
Activity
The activity of a sample is the average number of disintegrations per second its unit is the becquerel (Bq).
One becquerel is one decay per second.
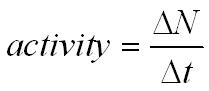
DN = change in number of undecayed nuclei
Dt = change in time in seconds
Decay constant l
The decay constant l is the probability that a nucleus will decay per second so its unit is s-1.
activity = decay constant x the number of undecayed nuclei
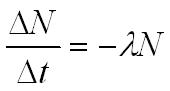
A = activity in becquerel (Bq)
N = the number of undecayed nuclei
l = decay constant (s-1)
Radioactive decay law
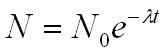
N0 = number of undecayed nuclei at t=0
t = time after t=0 in seconds
N = the number of undecayed nuclei at time t
l = decay constant (s-1)
Half life
The half life is the time for half the nuclei to decay.
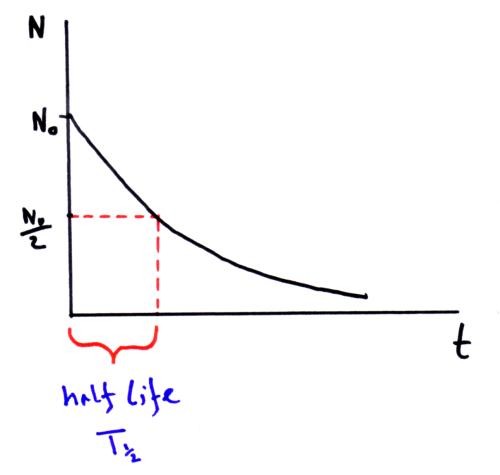
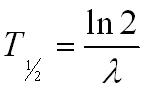
Half lives can vary from seconds (e.g. radon-224 half life = 55 seconds) to millions of years (e.g. potassium-40 half life = 1.3 x 109 years). This has implications for radioactive waste from nuclear power stations which will need to be stored safely for a very long time.
Radioactive dating
Carbon-14 has a half life of 5730 years. Once a plant or animal dies its carbon-14 content gradually decreases. Using the half life for carbon-14 and comparing the amount of carbon-14 in on ancient artifact with the amount of carbon-14 we would expect in a fresh sample today we can date an object.



